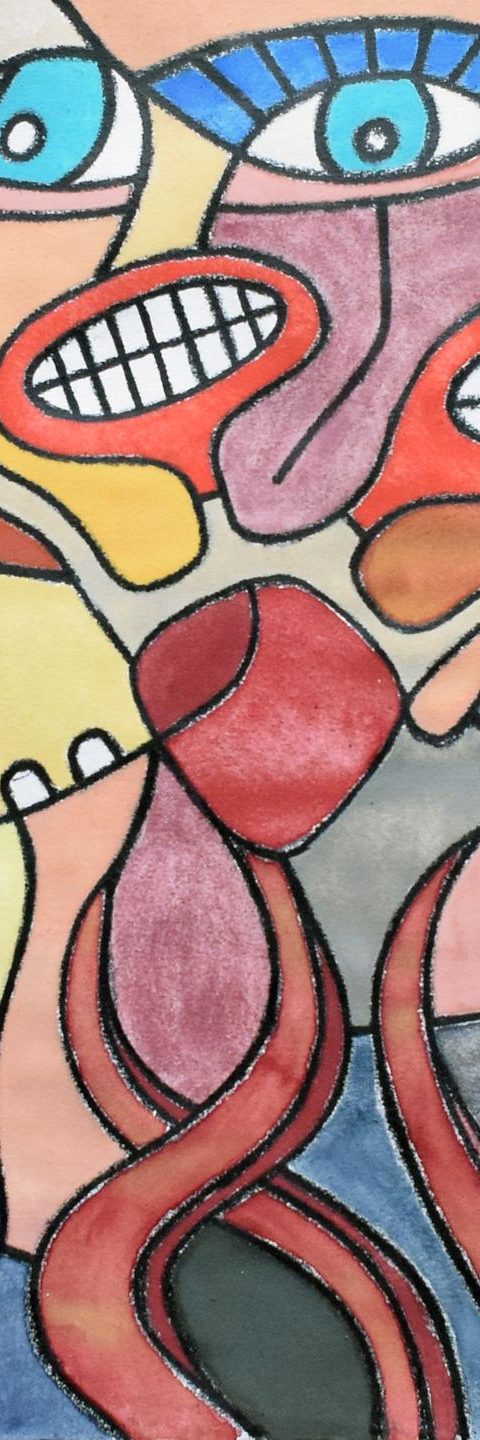Due to public liability concerns, our art studio remains a private space, closed to the general public. At this stage we can only offer our artworks for sale via our online shop. We’re happy to meet you somewhere in Moruya for a coffee or tea though.
Support FAQs
We currently do not sell prints, no. There are several reasons for this. One of the main reasons that we do not sell prints of our artworks is that the ink that is used in digital prints is not light fast, so it will begin to fade in a very short amount of time.
Next, paper quality. Watercolour paper is generally superior to that of digital printing paper. Digital printing paper on the other hand is often very smooth, something which we don’t really like. So watercolour paper has a much nicer texture and it is also generally a lot thicker than digital printing paper too.
We know that you generally get what you pay for. We think that prints won’t ever increase in value, but will more likely decrease. Real art on the other tends to be an investment for the future.
You are in luck. You can commission an artwork here.
We only use professional artist quality pigments (mostly from Schmincke). These watercolour pigments are usually very light fast, especially when compared to vegetable or soy-based inks/dyes.
In addition, van den hooven doesn’t just use any old artists pigments but rather has a strong preference for ‘inorganic’ pigments as opposed to ‘organic’ pigments. You can read more about this below.
You can therefore rest assured that your art investment will endure for a very long time to come…
Often when an artist wants to create a new direction they first make several rough/quick ‘study’ works in order to evaluate the potential for new compositions and check the feasibility/attractiveness of those before making a much bigger commitment in terms of art materials (and time).
This is generally done on small size scales first as it is much more economical that way (rather than potentially ‘ruining’ large amounts of expensive paper). From those very first initial works, the ‘ugliest’ ones are rejected, one learns what not to do, and a new direction is taken.
Several of these very early works by van den hooven don’t really make the cut for the main website, but they may nevertheless still interest art collectors and/or art investors because they show the progression or evolution of van den hooven’s art style over several years and the prices are also very reasonable.
A3
https://www.etsy.com/au/listing/1224757026/pop-art-style-original-illustrative
https://www.etsy.com/au/listing/1238690635/pop-art-style-original-illustrative
https://www.etsy.com/au/listing/815595410/van-den-hooven-internal-organs-body
A5
https://www.etsy.com/au/listing/1122790134/abstract-surrealism-organs-eyes-iris
https://www.etsy.com/au/listing/1136741923/abstract-surrealism-organs-eyes-iris
https://www.etsy.com/au/listing/1136741167/abstract-surrealism-organs-eyes-iris
https://www.etsy.com/au/listing/1136730431/abstract-surrealism-organs-eyes-iris
https://www.etsy.com/au/listing/1136731323/abstract-surrealist-artwork-flying
Some of our other previously sold artworks are listed here.
Several other artworks by the same artist are available on our secondary [Etsy] shop here.
Although please keep in mind this art is produced in a variation of styles and are therefore not considered consistent and homogenous enough of an art series to make it all available for sale on our main van den hooven online shop.
Nevertheless, we realise or recognise that some art collectors, art curators or art historians may appreciate the progression of and experimentation van den hooven has had with other art styles and subjects.
(note that we also make eco mouse mats, and for the time being it’s easier to maintain one shop rather brand everything separately so for the time being everything is bundled in together over there, almost like an factory outlet of art).
What’s the difference between ‘organic’ and ‘inorganic’ pigments?
What’s the difference you ask? We’re glad you asked, and we’d like to clear that up for you once and for all.
The difference is that ‘organic’ pigments are primarily composed of compounds containing the element carbon (and hydrogen). They may not even contain any metal elements at all, just mainly carbon.
Since van den hooven has a strong science background, most of the colours that van den hooven chooses to use today are inorganic pigments (as opposed to “organic” pigments). Why?

Inorganic pigments
Very basically, inorganic compounds are those that exclude carbon molecules. They don’t contain carbon. They are primarily composed of any of the other elements remaining in the periodic table.
Now, most substances (elements) in nature like to end up as compounds, since they are more stable that way, thermodynamically speaking. Some of them end up as very stable minerals crystals (for example silicates; for example the Earth pigments), many are oxides, some are sulfides, and so on.
The resulting bonding between metals and non-metal atoms in such compounds is known as “ionic bonding”. Ionic bonds are stronger than covalent bonds because they involve the transfer of electrons rather than an equal sharing of electrons (and something about ideal bond angles). And since ionic bonds are generally stronger than covalent bonds, more energy is required to dissociate ionic bonds than covalent bonds.
Furthermore, some of these inorganic compounds form what are known as “ionic crystal lattices”: a regular repeating arrangement of ions spread out in all three dimensions (no I’m not making this up) which reinforces things even further. It’s all about the bonding.
That’s essentially why plastic melts/degrades upon heating and things like rust and salt don’t. More on the significance of this below.
So for example, does Ayers Rock ‘fade’? No. It’s basically the same colour it’s always been. And it’s been bathing in high-intensity UV light for some 500-odd million years or so.

Organic pigments
“Organic” pigments on the other hand, are primarily composed of carbon and hydrogen, with some other elements such as oxygen and nitrogen sometimes thrown in.
All organic carbon compounds have what is known as covalent bonding, whereas as stated above, most inorganic compounds have ionic bonding. So in a nutshell, carbon compounds, the so-called “organic compounds”, generally have significantly weaker molecular type of bonding mode than inorganic compounds.
Worse, many synthetic organic pigments that do contain metals (like phthalocyanine) are in fact not entirely covalently bonded. The lone central copper (or perhaps nickel) atom here instead forms something called an “organometallic complex”, exhibiting a special type of “coordinate bond”. Complexes are biodegradable. These complexes are not to be confused with chelates (eg chlorophyll, the green pigment of plants) or coordination complexes. And the bonds binding the singular metal atoms contained in this lot are weaker still. Well they certainly are if the surrounding oragnic molecule breaks apart for whatever reason. Then there are things called ligands…
[but here’s where my knowlege of chemistry gets a bit rusty, because having only done three chemistry subjects, I branched off and studied materials (solids) instead, whereas chemists are always going on about liquids…]
Now here’s the scary thing. The thing is, carbon-carbon bonds can and do break apart. Organic compounds are known to decompose in ultraviolet light. UV light essentially attacks these bonds and the resulting process is known as photodegradation or photooxidative degradation (via chain scission).
So if you ever see a horrible pthalo green or pthalo blue colour in something claiming to be a van den hooven, you instantly know it’s a fake.
To put this into numbers, lattice dissociation energies calculated for ionic [inorganic] compounds typically fall in the range of 600–4000 kJ/mol (some even higher), whereas covalent bond dissociation energies are typically between 150–400 kJ/mol for single bonds.
Summing up, essentially, inorganic pigments/compounds contain more of the good bits (transition metal elements) and less (or no) carbon + hydrogen.
So we, as former scientists, already know that inorganic pigments are generally-speaking the least likely to break down, decompose or fade (with few exceptions) because they are fairly basic, stable elemental compounds. Thus, they tend not to degrade in sunlight.
We don’t even really need to do any light resistance pigment testing, because even if an inorganic pigment does fade, which is unlikely, the chemical information is still there. Unlike an organic pigment, which becomes a complete carbon mess.
So who knows? In the far future, it would be possible to scan the composition of elemental residue over a van den hooven artwork in like a two-dimensional scanner, and because each artwork contains a significant amount of different transtition metals compounds, have it completely restored.
Conversely, in one hundred thousand years from now, you won’t know the remnants from one organic pigment from the other on an artwork made with synthetic organic pigments, and that’s why we primarily choose inorganic pigments instead wherever possible.
If this interests you further, you can read more about the molecular chemistry of synthetic organic art pigments here and here. For more information about chemical bonding in general, might I suggest the LibreTexts chemistry website. Lots of visual resources to be found about bonding there.
https://en.wikipedia.org/wiki/List_of_inorganic_pigments
https://en.wikipedia.org/wiki/Category:Organic_pigments
Furthermore, van den hooven has a preference for “single pigment” pigments as opposed to “mixed pigment” pigments. There are a few reasons that van den hooven has decided to take this approach to making art:
- Pure colours.
The main reason is that single pigments are more vibrant than colours mixed together from two or more other pigments. For example, RGB has three colours (red, green and blue). CMYK has 4 colours (cyan, magenta, yellow and black). The van den hooven palette contains 64 or more individual pigments, each with their own characteristic hue. - More consistent colour palette.
It’s easier for van den hooven’s style to shine through because the same colour palette is repeated. - It significantly easier for future art restorers and conservators to repair any damage if they know the exact pigments that were used.
The artworks are offered in a range of sizes.
The smallest artworks are A4-sized (210x297mm).
The largest artwork(s) in the collection measure 97cm x 55cm.
Many of these artworks have a more ‘panoramic‘ nature than the more common/traditional 3:2 or 4:3 aspect ratio or conventional ISO paper size designations (with 1 to 1.414 height-to-width ratio).
We feel that an aspect ratio closer to 16:9 widescreen format is more modern, more contemporary, more fashionable. More suited to the way humans see things naturally.
We define a ‘panoramic’ artwork as being at least twice as wide as it is tall. Or in the case of a vertical panoramic artwork, at least twice as tall as it is wide.
Empowering creativity sustainably, our zero waste arts business envisions a world where artistic expression harmonizes with environmental responsibility. Through mindful sourcing, eco-friendly practices, and innovative upcycling, we aspire to inspire a community that values artistry without compromising the planet’s well-being.
We here at van den hooven believe that fur belongs on the animal. We refuse to either buy or use sable paintbrushes in particular, as we know they contribute to ongoing animal cruelty.
We honestly find it rather disheartening as we learn more about how brushes are manufactured and how the fur in them is harvested. Using animal by-products is one thing (for example paper sizing), but is quite another to obtain primary products from them (brushes).
What brushes does van den hooven currently use? We have bought synthetic/pony and goat hair brushes in the past (and still use them so as not to be wasteful). We own one squirrel brush (which quite honestly is too soft anyway, it’s not stiff enough and so we won’t be getting any more of those in future).
The trouble is that to our knowledge many manufacturers of synthetic toray and taklon brushes also produce and sell a whole range of kolinsky sable brushes, which indirectly supports animal cruelty.
Thus, we’ve already made a pledge to buy synthetic brushes in future (taklon). Most likely Roymac revolution quill mop made by Micador, which also uses a 100% FSC® certified handle. Because their whole business does not centre and revolve around selling sable brushes.
Copyright
Copyright protects the subject matter from being copied or used in certain ways without the copyright owner’s permission. As such, copyright is a mechanism for artists to protect and monetise their creativity.
Copyright laws protect electronic versions of artworks as much as ‘real’ or material work such as painting or photograph.
Piracy
With digital technology, it is very easy for people to copy or use other people’s work without permission. This happens a lot with music or film on the internet.
Everyday, people use the internet to copy and share other people’s work for free, and without permission. This is called piracy.
The artistic creations showcased on this website are the exclusive intellectual and artistic possessions of their respective creator, van den hooven. Reproduction in any form –in any country– without prior permission is strictly prohibited and constitutes a criminal offense.
We ask that you not download, copy or distribute any digital reproductions of van den hooven artworks without our prior express consent.
Moral rights
In addition to copyright, there are legal obligations to attribute creators and treat their work with respect. These creators’ rights are known as ‘moral rights’.
Moral rights are granted to creative professionals including visual artists. Moral rights are the right to be correctly attributed, the right not to be falsely attributed, and the right of integrity to prevent derogatory treatment of the material. Moral rights are separate to copyright and cannot be given away, however you can consent to activities that may infringe your moral rights.
Moral rights are personal legal rights of creators in respect of certain copyright material, that exist separately to copyright. They are rights which protect the creative reputation of certain artists, and unlike copyright they cannot be given away.
Moral rights protect the personal relationship between a creator and their work even if the creator no longer owns the work, or the copyright in the work.
The publisher of a work of art must publish the artist’s name along with the reproduction. The illustrator or artist is entitled to object to any derogatory treatment of their work. Moral rights are not transferable and are non-economical (ie. they cannot be sold).
Creators have moral rights even if they do not own copyright in their work. They cannot sell or completely waive their rights, but they can give consent for certain things that may otherwise breach their moral rights.
Moral rights cannot be bought or sold or given away; however after the creator passes away, the moral rights can be exercised by the personal representative of the creator, for example, the executor or administrator of the creator’s estate.
The moral right of attribution and the moral right against a false attribution all continue in force until copyright in the artistic work expires – usually 70 years following the creator’s death.
Intellectual Property
The term ‘intellectual property’ or ‘IP’ refers to the property rights that arise in the outcomes of creative and intellectual processes, such as artworks, designs and inventions. These rights include copyright and moral rights, and are legal tools that practitioners can use to protect their work from unauthorised use, to protect their reputation or brand, and generate income.
We assert that it is fair for artists and designers alike to be entitled to control the use of our intellectual property.
About Fair Dealing
The copyright law of Australia is governed by Copyright Act 1968 and operates nationwide. This Act and subsequent amendments gives practitioners control over the reproduction of their work. This includes all artistic creators, not just those who may consider themselves ‘professional.’
Currently it allows for certain limited exceptions under the general heading ’fair dealing’. The main exceptions to copyright infringement are the use for the purposes of:
· review or criticism
· research or study
· news-reporting
· parody or satire
· judicial proceedings or professional advice
It must also be ‘fair’. What is fair will depend on all the circumstances, including the nature of the work, the nature of the use and the effect of the use on any commercial market for the work.
If a creator’s moral rights are found to have been infringed, the court can order any of the following:
· a public apology;
· a declaration of infringement;
· payment of money for the harm suffered (damages);
· the person to stop the infringement (an injunction); or
· that any false attribution be publicly corrected;
· that any derogatory treatment ceases or is reversed (as appropriate).
What payment methods are accepted?
We have several payment methods available on our online shop.
We accept all major debit and credit cards including VISA, Mastercard, American Express (Amex), Diners Club card, Discover, UnionPay International (UPI), Japan Credit Bureau (JCB).
We also accept the following additional pament methods: Paypal, Paypal Express, Google Pay, Apple Pay (only available on Apple devices), Airwallex and direct bank transfer (IBAN/SWIFT).
Cash (in Australian dollars) is only an accepted payment method using our local pickup/collection delivery option.
Do you accept any buy now, pay later (BNPL) services?
For the payment options listed above, 100% of the payment is due at the time of purchase. We do also currently accept AfterPay (up to the maximum amount of AUD$2000) and Klarna payment/repayment options. These are enabled at the time of checkout.
What payment methods aren’t accepted?
We do not currently accept cheque payments, Bitcoin and other similar digital cryptocurrencies.
In essence, an NFT is a digital certificate of ownership, almost always bought and sold using cryptocurrency, to which any digital file – a jpeg image file, a video, a song – can be attached.
With a simple google search, anybody can find and download the file associated with an NFT, for nothing, and store it on their phone or computer. But only the owner has the right to sell it.
Anyone can view the individual images—or even the entire collage of images online for free. So why are people willing to spend millions on something they could easily screenshot or download?
We’re not really sure why to be honest. It could be just a fad. We’ll look into it, but we’re not really interested in producing or selling NFT are at this time, no.
The main reason is that van den hooven prefers to make real analogue art rather than digital art. The other reason is that blockchain tech has a rather large environmental footprint.
In short, no. We now accept Bitcoin or other cryptocurrencies to pay for our art, yes.
This is a somewhat reluctant move, and we still prefer other more traditional payment methods (read our full thoughts here).
However in the interest of encouraging new orders and lowering the barrier to entry for new and established art collectors alike, we recently decided to accept Bitcoin and other cryptocurrencies via our online store.
An art sale is an art sale, and we’re not really in a position to be fussy about accepting various alternative payments. Quite the opposite. We want to make it easier for people to buy our art, not harder. Neither do we think we really have the power to influence the entire crypto market.
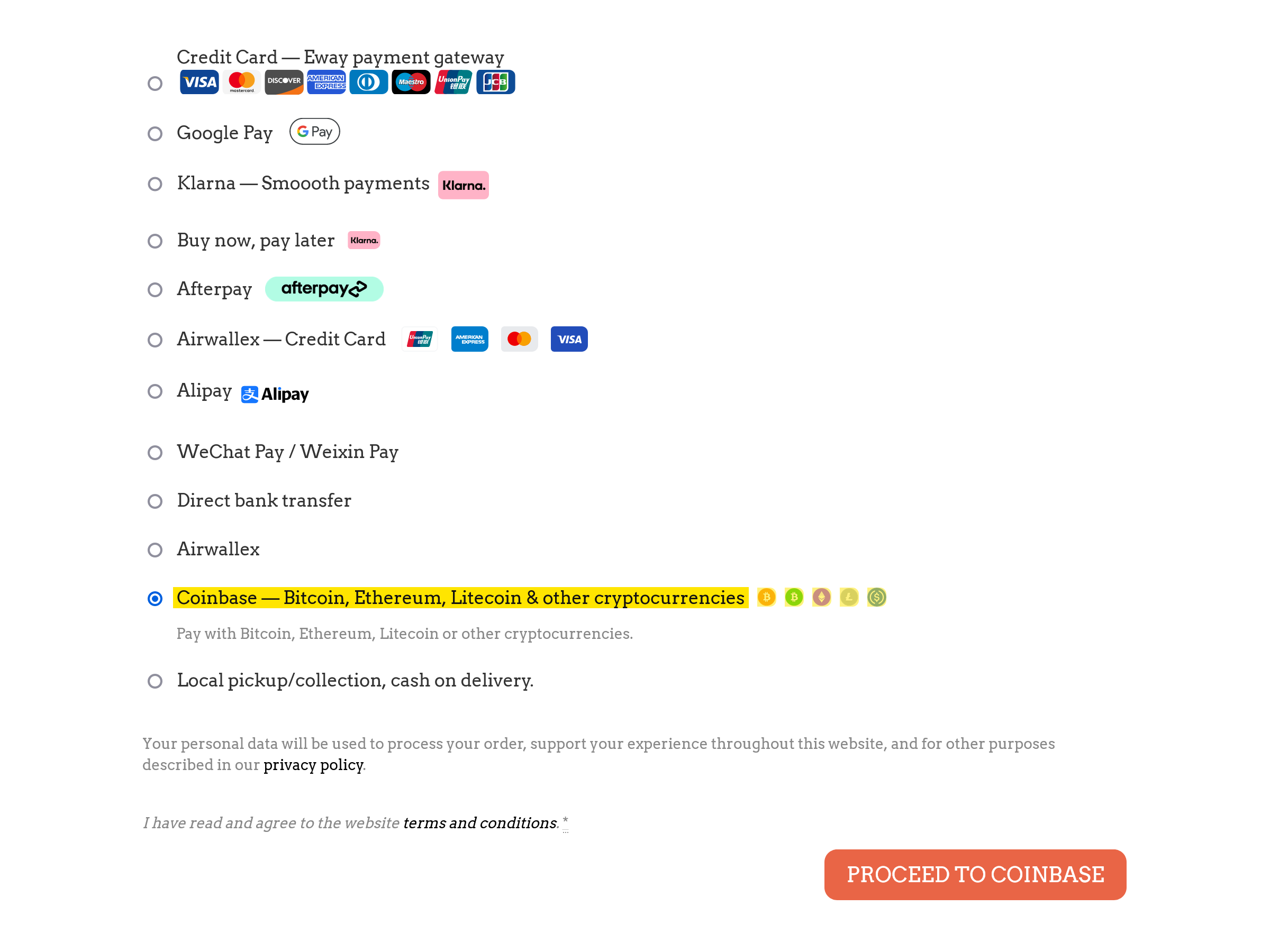
Please select the “Coinbase” payment gateway at the time of your checkout. From there click the “proceed to coinbase” button to finalise art transaction.
![]()
We believe that getting a refund shouldn’t feel like having a wisdom tooth extraction. Quite the opposite. It should be quick, easy, painless and “no questions asked”.
Besides, it really isn’t in our best interests if you don’t like our art (for whatever reason). We obviously want our art buyers to speak about us in a positive light. So if you aren’t happy with your art purchase, just let us know within 30 days, return it to us, and –provided the artwork isn’t damaged– you’ll automatically be entitled to a full refund. We think that is only fair.
Consumers have the right to return a product if they think there’s a problem. The product does not have to be in its original packaging, but a business is entitled to ask consumers to provide some form of proof of purchase, such as a receipt.
If the business confirms that the product does have a problem, it must reimburse the consumer for any reasonable return costs they have already paid.
Consumers should keep receipts for postage or transport costs so that they can be repaid by the business.
If the business finds that the product does not have a problem, it can make the consumer pay the collection and inspection costs. To do this, the business must give the consumer a reasonable estimate of these costs before collecting the product.
Under Australian Consumer Law, a store does not have to give a refund or replacement if a customer simply changes their mind about a product. Generally the customer is only entitled to a refund or replacement for a major problem with a product covered by consumer guarantees.
A consumer is generally entitled to receive any refund in the form of their original payment. For example, if they paid for an item with a credit card, it is reasonable for the seller to give the consumer a credit card refund. It is misleading for a seller to insist that a refund
be issued as store credit.
Under Australian Consumer Law, most products and services bought in Australia (from 1 January 2011) come with automatic consumer guarantees that the product or service you purchased must be of acceptable quality.
Australian businesses must provide these automatic guarantees regardless of any other warranties they give or sell you. If a business fails to meet any of these guarantees, you have the right to a refund.
Our Privacy Policy explains how we handle your personal information. We’ll update this policy if and when our information handling practices change.
Our Privacy Policy outlines what kinds of personal and sensitive information we collect, why we collect this information, and how we handle it.
Only Australian Government agencies and organisations with an annual turnover more than $3 million have responsibilities under the Privacy Act, subject to some exceptions.
As a small business operator, we are more or less exempt from even declaring or having Privacy Policy. Some small businesses and not-for-profits have made a public commitment to good privacy practice and opted-in to the Privacy Act. This allows them to benefit from any increase in consumer confidence and trust that may be derived from operating under the Privacy Act.
Nevertheless, we believe that entities should take appropriate responsibility for ensuring that their information handling practices are fair and not harmful. There should be greater protections for personal information before it is used in ways which have high privacy risks. Individuals need more transparency about what is being done with their information and more control over what happens with it.
Essentially, we only collect personal information about you in order to be able to process and send your order(s). We do not share or sell any information to any 3rd party organisations.
We never publicly disclose, identify or publish those individuals who have bought our artworks. We feel this information is strictly confidential information and that it should remain private.
If you make an artwork purchase on our site, our payment gateway providers (banking merchants such as woocommerce and paypal) then retain and store the information associated with that transaction; details such as your name, your paypal address, your debit or credit card number. Those corporations will have their own privacy policies.
The only other people that see your name and address on an order are obviously postal workers themselves, who need to see who and where they are delivering merchandise to.
What else? Because we essentially do our own website design and maintenance, we can tell you that all internet host providers monitor and store website traffic data. In addition, your own internet Service Provider (ISP) also stores this data (although that is beyond our control because that happens over at your end).
These are known as “access logs”. This information includes IP addresses of computers that have visited our site, the webpages visited and the order in which those pages were visited, dates and times associated with loading these pages, your browser type and version (whether you use mozilla firefox or chrome for example) and your operating system type (eg windows or mac). Other than that you are generally pretty anonymous and we can’t really know who is the person visiting any of our webpages at any given time. Besides, we are generally too busy to ever check this out (honestly). We do have access to some limited visual statistics on our web hosts’s control panel, but these are from some of our other sites as well and it’s all jumbled together, hence all that info is practically useless to us.
The more detailed access logs themselves are generally for debugging purposes only. Nobody in their right mind would go through these as they are non-visual and extremely boring to look at. Thus, we most certainly have better things to do (such as creating art).
Our web page visitor statistics and access logs are only available online for us to see, but they are not made public. Besides us, only our web host has access to this information.
To improve your experience on our site, we may also use ‘cookies’. Cookies are pieces of information that a website can transfer to an individual’s computer hard drive or hand held device for record keeping. Cookies can make websites easier to use by storing information about your preferences on a particular website. The information is used by us to help improve our website by understanding how it is used and effective communication methods for diverse audiences. We do not attempt to identify individual users in any way.
We have checked this site and it uses some (minimal) cookies in order to save a few of your preferences such as: your preferred shop currency; and to remember whether you’ve added something to the cart. There is also one for Google’s now invisible reCAPTCHA v3 anti-spam bot on our contact page. Last but not least, our cookie notice stores information about your cookie preferences when you select ‘okay’.
We did not intentionally set out to create a website that uses cookies. Frankly speaking, we’d rather not deal with annoying cookie popup windows and GDPR compliance. In order to improve our user experience and sell to European markets though, it has to happen.
We do not track anybody using cookies and we certainly don’t use any so called “third party” cookies for advertising or other nefarious purposes.
You can learn more about what cookies are here:
https://en.wikipedia.org/wiki/HTTP_cookie
https://en-au.wordpress.org/about/privacy/cookies